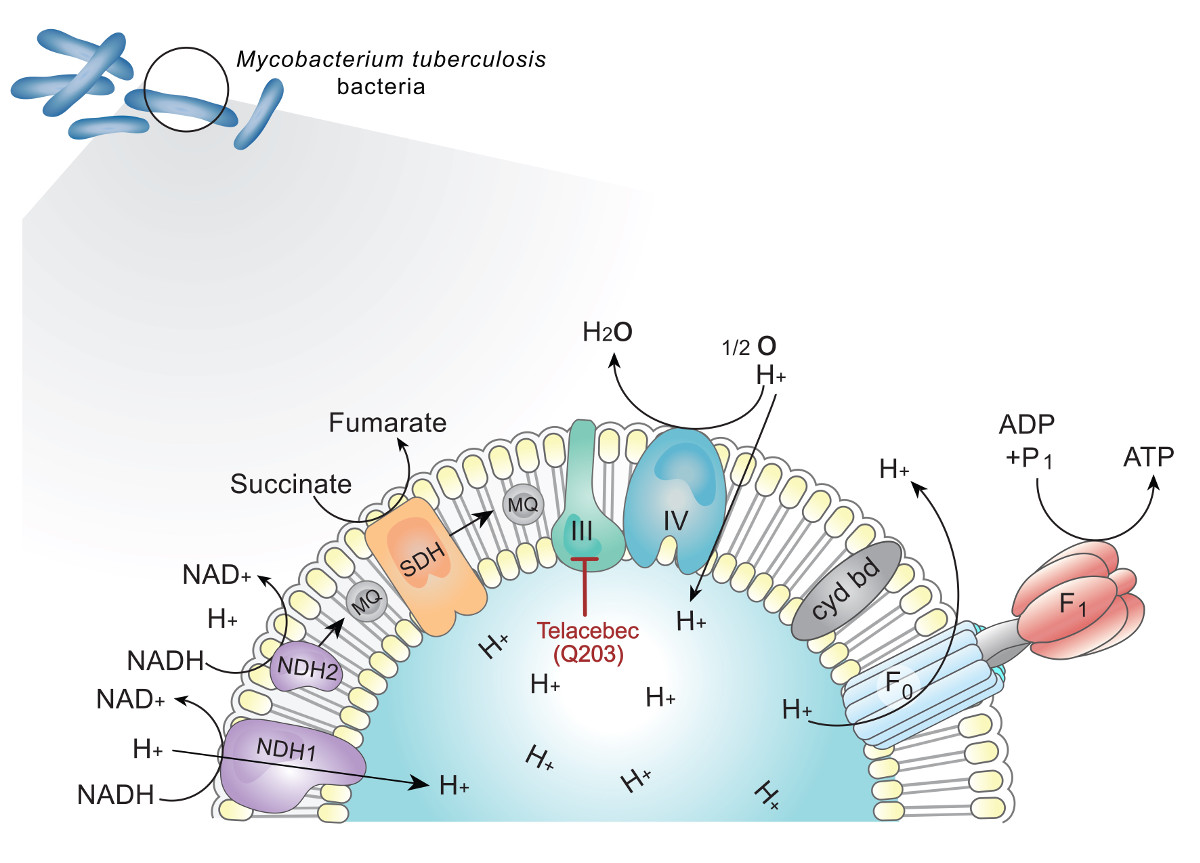
Telacebec (Q203) is a first-in-class drug candidate against Mycobacterium tuberculosis infection. Telacebec (Q203) targets cytochrome bc1 complex and shows potent activity against multi-drug resistant tuberculosis (MDR-TB). Tuberculosis (TB) is a significant worldwide health problem and its drug resistant form, in particular, is of growing concern since it has limited options for treatment. However, no new drugs have been approved for TB in the forty years prior to 2012. Shortcomings of recently approved drugs for the treatment of drug resistant TB [bedaquiline (SirturoTM, Janssen, approved by the FDA in 2012) and delamanid (DeltybaTM, Otsuka, approved by the EMA in 2013)] include the unavailability of a clear combination dosing regimens, and/or safety concerns (QTc interval prolongation, abnormal hepatic function and/or increased risk of death). Thus, there is a need for new therapies with a novel mechanism of action that offer efficacy against both drug sensitive and drug resistant TB with an acceptable tolerability profile, to provide more options for combination regimens in the fight against TB. In particular, a ‘universal regimen’ made of new drugs is needed to treat tuberculosis regardless of its drug resistance status in order to fight tuberculosis in every corner of the world.
Telacebec (Q203) is one of the first candidates getting close to forming new universal regimen. Telacebec (Q203) is an orally active small molecule drug candidate that blocks Mycobacterium tuberculosis growth by inhibiting cytochrome bc1 complex, leading to the depletion of adenosine triphosphate (ATP) synthesis of M. tuberculosis. Telacebec (Q203) shows good synergy with bedaquiline in murine chronic infection model, indicating promising potential for new drug regimen.
Telacebec (Q203) was found effective with good dose response in the phase 2A, early bactericidal activity (EBA) study with drug susceptible TB patients.
Telacebec(Q203) has received Orphan Drug Designation and Fast Track Designation from the U.S. FDA.
Telacebec(Q203) also has been found effective against buruli ulcer (Mycobacterium ulcerans), a chronic, necrotizing disease that affects skin and sometimes bone and can lead to permanent deformity and long-term disability.
Publications
1. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis, Nature medicine, 19(9), 1157-1160, 2013, published on Aug. 4th, 2013. (Pubmed)
2. Lead Optimization of a Novel series of Imidazo[1,2-a]pyridine Amides Leading to a Clinical Candidate (Q203) as aMulti- and Extensively-Drug Resistant Antituberculosis Agent, Journal of Medicinal Chemistry, 57, 5293-5305, 2014; (May, 28) (Pubmed)
3. Synthesis and structure-activity studies of side chain analogues of the anti-tubercular agent, Q203, European Journal of Medicinal Chemistry, 125, 807-815, 2017; (Jan. 5th) (Pubmed)
4. Synthesis and structure-activity relationships of novel fused ring analogues of Q203 as antitubercular agents, European Journal of Medicinal Chemistry, 136, 420-427, 2017; (Aug. 18th) (Pubmed)
5. Targeting the Mycobacterium ulcerans cytochrome bc1:aa3 for the treatment of Buruli ulcer, 9(1), 5370, 2018; (Dec. 18th) (Pubmed)
6. Telacebec for ultra-short treatment of Buruli ulcer in a mouse model, 10.1128/AAC.00259-20, 2020; (Mar. 23th) (download)
7. Telacebec (Q203), a New Antituberculosis Agent, N Engl J Med 382;13, 2020; (Mar. 26th) (download)
8. Telacebec, a Potent Agent in the Fight against Tuberculosis: Findings from a Randomized, Phase 2 Clinical Trial and Beyond, Am J Respir Crit Care Med. 2025 Aug;211(8):1504-1512. (Mar. 18th) (Pubmed)
Clinical Trials
1. A Dose-Escalation Study to Evaluate Safety, Tolerability and Pharmacokinetics of Single Doses of Q203 in Normal, Healthy, Male and Female Volunteers (NCT02530710)
2. A Dose-Escalation Study to Evaluate Safety, Tolerability and Pharmacokinetics of Multiple Doses of Q203 in Normal Healthy Male and Female Volunteers(NCT02858973)
3. A Phase 2 Study to Evaluate Early Bactericidal Activity, Safety, Tolerability, and Pharmacokinetics of Multiple Oral Doses of Telacebec (Q203) (NCT03563599)
4. Telacebec (T) Treatment in Adults With Buruli Ulcer (BU). (TREAT-BU) (NCT06481163)
Related News
1. FDA grants orphan drug designation to Qurient’s ulcer treatment (FDA)
2. Qurient Announces Publication in New England Journal of Medicine of Phase 2 Data for Telacebec, a First-in-Class Antibiotic for the Treatment of Tuberculosis (businesswire)
3. Qurient Co. Ltd. and TB Alliance Announce Exclusive License Agreement for Telacebec (Q203), a New Anti-Tuberculosis Agent (PR newswire)
 Telacebec (Q203)
Telacebec (Q203)