Q301 is the first topical leukotriene inhibitor for atopic dermatitis under phase 2b developmentAtopic dermatitis (AD), also called eczema, is a chronic, pruritic, and inflammatory skin disease that occurs most frequently in children but that also affects adults. The hallmarks of AD are a chronic, relapsing form of skin inflammation and a disturbance of epidermal-barrier function that culminates in dry skin.
Most commonly used topical treatment for AD is corticosteroids, which carry serious adverse effects prohibiting continuous use of the drugs. Despite recent approval of non-steroid topical agents for AD, development of therapeutic agents with novel mechanism of actions is still needed as complete response rate of the available agents is limited and understanding of disease progress mechanism is still at large.
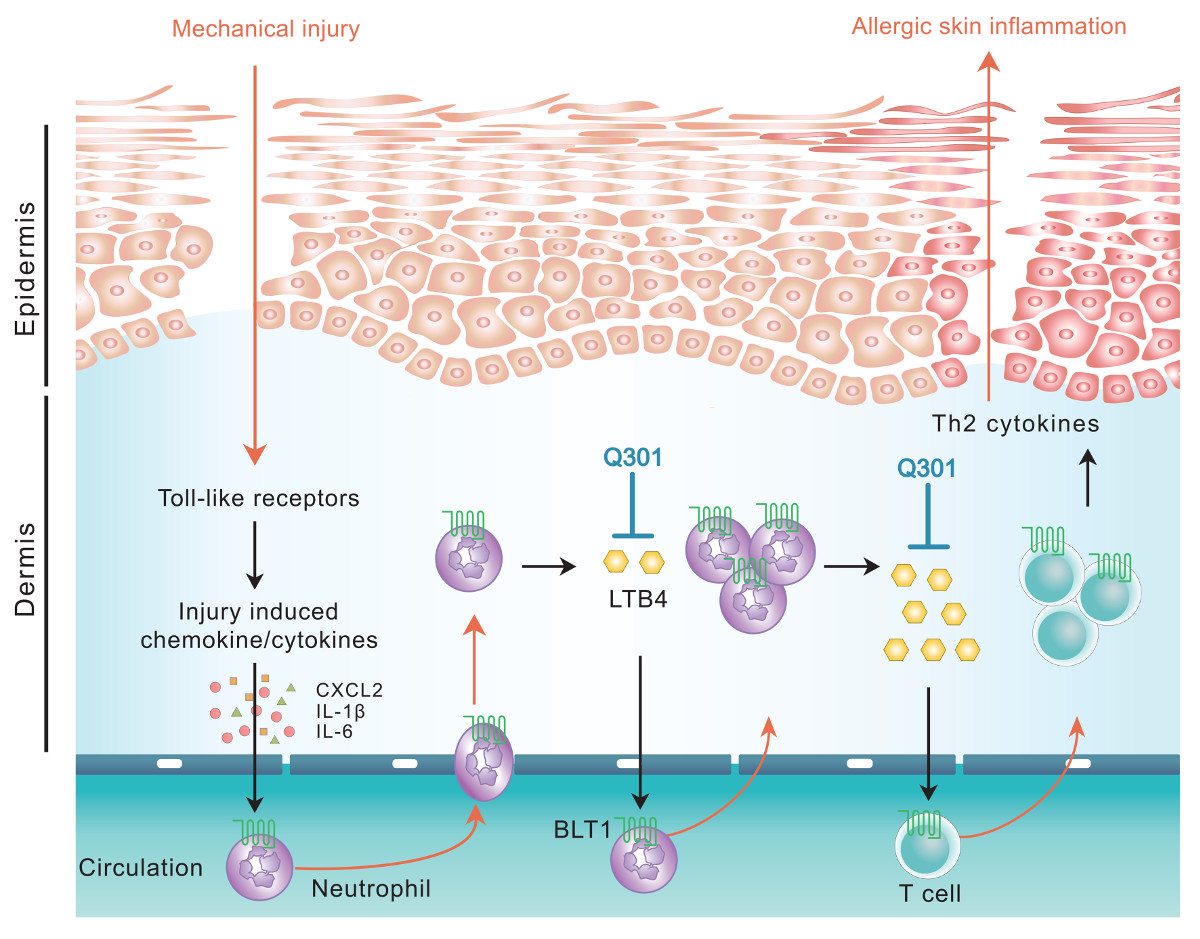
Leukotrienes, particularly leukotriene B4 (LTB4), has been reported to play significant roles in AD disease progression by recruiting inflammatory cells to the lesion, nevertheless there has not been a leukotriene inhibitor developed for treatment of AD. Q301 is the first topical agent with 5-lipoxygenase (5-LO) inhibitor, zileuton as an active pharmaceutical ingredient under phase 2 clinical development for AD.
Q301 achieved proof-of-concept in a phase 2A study under U.S. IND carried out with 60 moderate to severe AD patients based on IGA score. In this study, about 30% Q301 treated patients show clear to almost clear (IGA grade 0 or 1, respectively) with at least two degrees of improvement, which is significantly better than placebo treated patients group showing 4% of patients achieving the same degree of improvement.
Q301 is currently under phase 2B study with 240 mild to moderate AD patients in the U.S. based on IGA score. Adolescent population (age 12 and over) is included in this study based on supportive safety findings in the phase 2A study.
Poster
1. Q301 (Zileuton) Cream Demonstrates Superiority to Vehicle in Improving Atopic Dermatitis: Results From a Phase 2A Trial(2019 AAD annual meeting)
Clinical Trials
1. Safety and Efficacy Study of Q301 in Moderate to Severe Atopic Dermatitis Patients (NCT02426359)
2. Safety and Efficacy Study of Q301 in Mild to Moderate Adolescents and Adults Atopic Dermatitis Patients (NCT03571620)
 Q301 : Topical 5LO Inhibitor
Q301 : Topical 5LO Inhibitor